Sensational Info About How To Prevent Corrosion In Iron
Corrosion inhibitors are chemicals that react with a metal surface to.
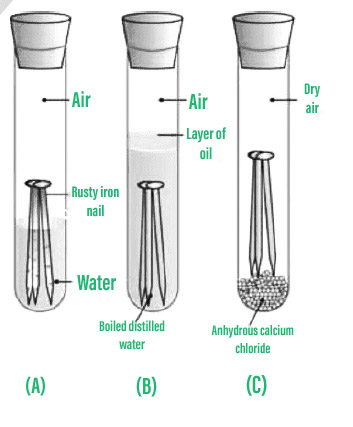
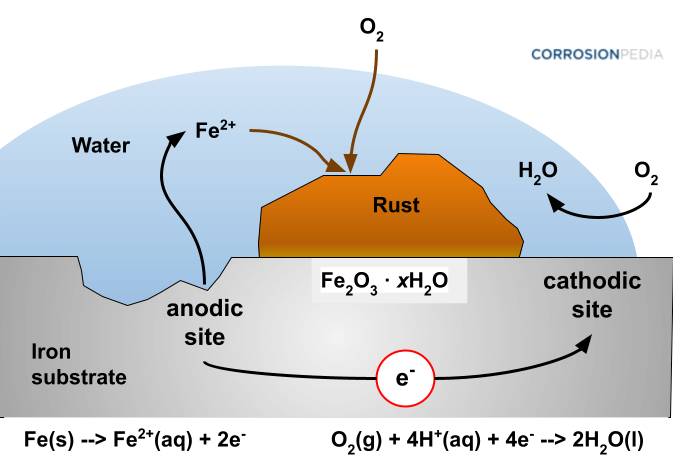
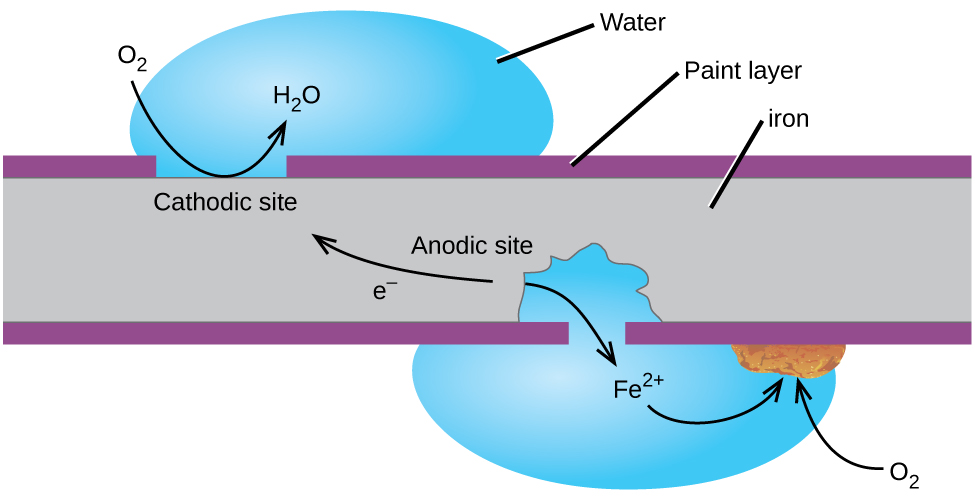
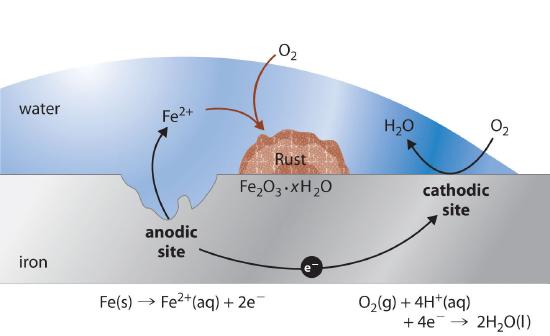
How to prevent corrosion in iron. An understanding of the activity series investigated in experiment 6 suggests that one way of preventing the corrosion of iron is to protect it with a more active metal. Oxidization (a chemical reaction between iron and oxygen) results in rusting on the surface of the metal. How does eoncoat provide internal pipeline corrosion protection?
As steam consumption is more feed pump will be continuous running. If the large amount of leakage, boiler. We would like to show you a description here but the site won’t allow us.
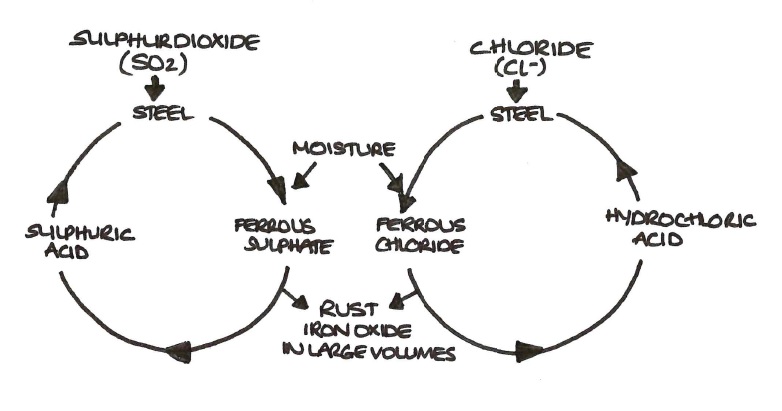
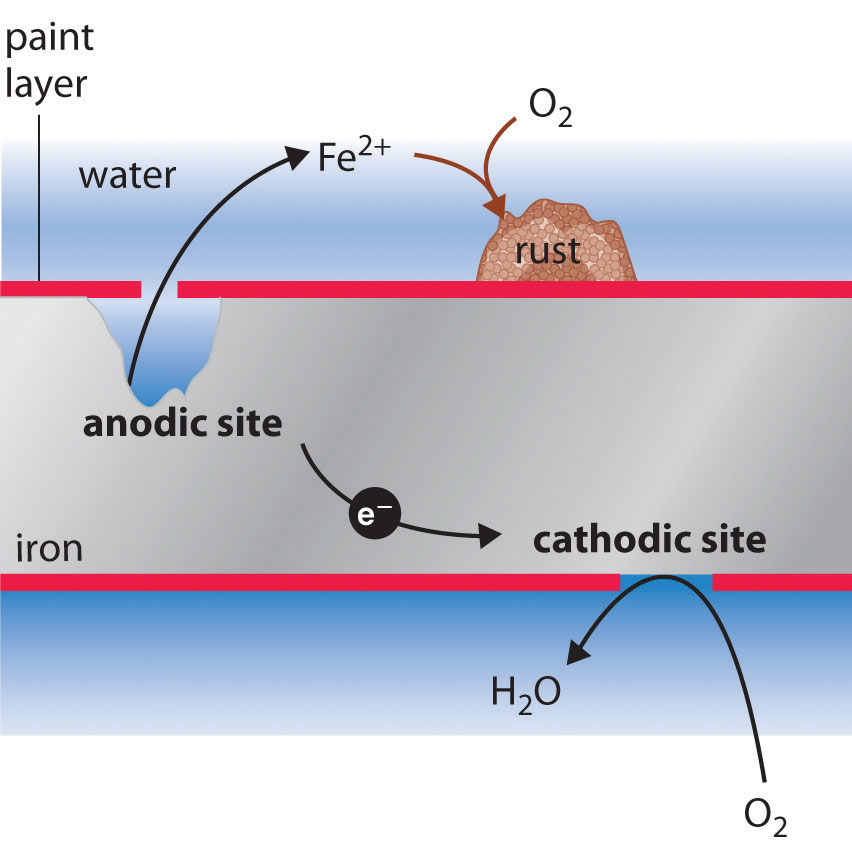
Galvanizing is the process by which a metal, like iron, is coated with another metal, such as zinc. This coating of what is called a “sacrificial metal”. Corrosion can also happen when metals like steel are placed under too much stress causing the material to crack.
The simplest component to remove is the metal surface. Iron tends to rust faster in the sea, due to the presence of various salts. 1)many different types of coatings can be applied to the surface of the exposed metal in order to prevent corrosion.
Berkut34 | dreamstime.com) when stainless alloys are welded, the formation of chromium. The most common type of iron corrosion occurs when it is. Excessive feed water consumption from cascade tank or feed water tank.
But oxidization is just one example of corrosion. Higher ph inhibits the corrosion of iron. Oxygen can be excluded by storing the.













/GettyImages-548553969-56a134395f9b58b7d0bd00df.jpg)